Ocher
  |
- Ocher appears one ~270,000 year old European cave drawings and ~700,000 year old Asian grave sites.
- Variations in the color of ocher derive from the degree of hydration and from which other minerals are present. Reds darken with time to browns, due to acquisition of waters of hydration or a change in the oxidative state of iron.
- Hematite is a hard, brittle black mineral of ferric oxide: Fe2O3.
- If ground fine, a red ocher results that lacks any waters of hydration (anhydrous iron oxide). This makes the Venetian red pigment and the sinopia of ancient to medieval Greek and Roman times.
- Goethite is iron hydroxide (O=Fe–OH), complexed with variable numbers of waters of hydration: FeO(OH) • (H2O )n.
- Brown ochers have few waters of hydration.
- Adding waters of hydration shifts the ocher to a striking yellow color; with time, the waters are naturally lost, causing the color to return to brown.
- Ancient Phrygians, such as Kings Giordas and Midas, used abundant yellow umber to dye their fabrics, and dust from their robes would have turned all they touched to a golden color.

Yellow ocher from Roussillon.
- Ancient Phrygians, such as Kings Giordas and Midas, used abundant yellow umber to dye their fabrics, and dust from their robes would have turned all they touched to a golden color.
- Limonite is a rock that is a matrix of ferrous minerals plus various other metals.
- Manganese oxide darkens the iron hydroxides, and is present in ochers mined in Umbria and near Sienna that are a dark brown.
- Raw umber and raw sienna can be baked, driving off waters of hydration and converting iron hydroxide to iron oxide, yielding the redder burnt umber and burnt sienna.
- Magnetite (Fe3O4) is the common black ore that is resistant to rust and that can be magnetized.
- Bluing is a process that causes an iron surface to become Fe3O4 and thus become resistant to further corrosion.
- Hematite is a hard, brittle black mineral of ferric oxide: Fe2O3.
- Human uses of iron oxides:
- Body ornamentation with ocher has been widespread in time and geography; Newfoundland Native Americans greeted French explorers in ocre, lending them the term "redskins" forevermore.
- FDA #172 dye (either red, black or yellow), permitted in small quantities in sausage casings and hard and soft candies, mints and chewing gums.
- CAS 1309-37-1: cosmetic pigment for nail polish, lipstick and lotions.
These blogs were inspired by a 2017 show at the Indianapolis Museum of Art called CSI: Chemistry of Color.
Egyptian Blue (Latin: caeruleum)
These images are ochre's chemical structure, a sample of the raw material and an example of its use from the museums's collection.
|
|
 |
- Egyptian blue is a much older technology than glass, first used ~3,000 BCE.
- The rectangular blue crystals contain 64% silica, 15% calcium oxide, 21% copper oxide, together with unreacted quartz and some glass.
- The pigment colors a variety of different media such as stone, wood, plaster, papyrus, and canvas, making cylinder seals, beads, scarabs, inlays, pots, and statuettes.
These blogs were inspired by a 2017 show at the Indianapolis Museum of Art called CSI: Chemistry of Color
Carmine (Cochineal)
These images are carmine's chemical structure, a sample of the raw material and an example of its use from the museums's collection.
|
Carminic Acid |
 |
 |
- The pigment was first used ~700 BCE.
- Carminic acid functions to deter predation by other insects.
- The acid is extracted from insect's body and eggs, and is mixed with aluminium or calcium salts to make carmine dye.
- In Mexico, cochineal scale (Dactylopius coccus) preys on the prickley pear cactus; the dye typically constitutes 17-24% of this insect's dry weight. Aztec and Mayan Indians used the dye extensively.
- Polish cochineal (Porphyrophora polonica) is native to the Polish-Lithuanian-Ukranian region, grows on the roots of knawel, a carnation, and contains 0.6% carminic acid.
- Today, carmine (which is NOT the USDA red #40) is primarily used as a colorant in food and in lipstick.
These blogs were inspired by a 2017 show at the Indianapolis Museum of Art called CSI: Chemistry of Color.
Vermilion (Cinnabar)
These images are vermilion's chemical structure, a sample of the raw material and an example of its use from the museums's collection.
 |
 |
 |
These blogs were inspired by a 2017 show at the Indianapolis Museum of Art called CSI: Chemistry of Color.
Prussian Blue (Pigment and Antidote)
These images are Prussian Blue's chemical and crystal structure, and a sample of the raw material.
 |
- Prussian Blue was discovered in 1706 when Johann Jacob Diesbach attempted to make Florintine lake (i.e. carminic acid extracted from cochineal, precipitated by alum, iron sulfate, and potash, to be used in painting). Providentially, he borrowed an inferior potash from Johann Konrad Dippel (an alchemist, born in Castle Frankenstein) that contained hexacyanoferrate because it was contaminated with processed blood; the iron sulfate precipitated the hexacyanoferrate, quite surprisingly making a brilliant and durable blue pigment. The pigment was promptly available commercially, and being used by painters in 1710.
- The name derived from Diesbach's country. Unpleasant associations shifted the name to Paris blue after World War I. Crayons have shifted to Midnight Blue. Turnbull blue has the same structure but differs in the size of the colloidal particles.
- Prussian blue is the pigment that has been used in blueprints and cyanotype prints since 1843, and as a paste used by machinists to reveal surface imperfections.
- Bluing - the fabric whitener - contains Prussian blue. By adding blue to yellowed cotton or linen, the eye perceives white - employing the opponent mechanism of color perception that is inherent to the function of the retina.
- Cyanide (German: Blausäure) can be chemically -and etymologically - derived from Prussian blue.
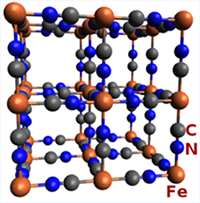 |
- Its exact composition (Fe3+4[(Fe2+(CN)6)4-]3) and structure was only recently determined and published in 1977. Curiously, the crystal contains both ferric and ferrous ions.
- The crystal structure is a palindrome written on a cube: iron(III), nitrogen, carbon, iron(II), carbon, nitrogen, iron(III) .....
- Being negatively charged, the crystal vigorously sequesters positively charged ions, particularly larger metals such as iron in the case of Prussian Blue. However, the crystal also binds toxic metals such as thallium and radioactive atoms, allowing the body to remove them rapidly, reducing their toxicity. This property is explained in this CDC poster.
References:
- H. J. Buser, D. Schwarzenbach, W. Petter and A. Ludi. “The Crystal Structure of Prussian Blue: Fe4[Fe(CN)6]3 • [H2O]x,” Inorg. Chem., 1977, 16, 2704-2710.
- Johann Leonhard Frisch. “Notitia Caerulei Berolinensis Nuper Inventi,” Miscellanea Berolinensia ad incrementum Scientiarum, 1710, 1, 377-378.
- Alexander Kraft. "On the discovery of Prussian Blue." Bull Chem History. vol 33. pp 61-67.
- George Pendle. "Colors / Prussian Blue." Cabinet Magazine, 2008.
These blogs were inspired by a 2017 show at the Indianapolis Museum of Art called CSI: Chemistry of Color.