Prussian Blue (Pigment and Antidote)
These images are Prussian Blue's chemical and crystal structure, and a sample of the raw material.
 |
- Prussian Blue was discovered in 1706 when Johann Jacob Diesbach attempted to make Florintine lake (i.e. carminic acid extracted from cochineal, precipitated by alum, iron sulfate, and potash, to be used in painting). Providentially, he borrowed an inferior potash from Johann Konrad Dippel (an alchemist, born in Castle Frankenstein) that contained hexacyanoferrate because it was contaminated with processed blood; the iron sulfate precipitated the hexacyanoferrate, quite surprisingly making a brilliant and durable blue pigment. The pigment was promptly available commercially, and being used by painters in 1710.
- The name derived from Diesbach's country. Unpleasant associations shifted the name to Paris blue after World War I. Crayons have shifted to Midnight Blue. Turnbull blue has the same structure but differs in the size of the colloidal particles.
- Prussian blue is the pigment that has been used in blueprints and cyanotype prints since 1843, and as a paste used by machinists to reveal surface imperfections.
- Bluing - the fabric whitener - contains Prussian blue. By adding blue to yellowed cotton or linen, the eye perceives white - employing the opponent mechanism of color perception that is inherent to the function of the retina.
- Cyanide (German: Blausäure) can be chemically -and etymologically - derived from Prussian blue.
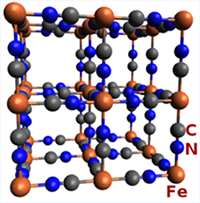 |
- Its exact composition (Fe3+4[(Fe2+(CN)6)4-]3) and structure was only recently determined and published in 1977. Curiously, the crystal contains both ferric and ferrous ions.
- The crystal structure is a palindrome written on a cube: iron(III), nitrogen, carbon, iron(II), carbon, nitrogen, iron(III) .....
- Being negatively charged, the crystal vigorously sequesters positively charged ions, particularly larger metals such as iron in the case of Prussian Blue. However, the crystal also binds toxic metals such as thallium and radioactive atoms, allowing the body to remove them rapidly, reducing their toxicity. This property is explained in this CDC poster.
References:
- H. J. Buser, D. Schwarzenbach, W. Petter and A. Ludi. “The Crystal Structure of Prussian Blue: Fe4[Fe(CN)6]3 • [H2O]x,” Inorg. Chem., 1977, 16, 2704-2710.
- Johann Leonhard Frisch. “Notitia Caerulei Berolinensis Nuper Inventi,” Miscellanea Berolinensia ad incrementum Scientiarum, 1710, 1, 377-378.
- Alexander Kraft. "On the discovery of Prussian Blue." Bull Chem History. vol 33. pp 61-67.
- George Pendle. "Colors / Prussian Blue." Cabinet Magazine, 2008.
These blogs were inspired by a 2017 show at the Indianapolis Museum of Art called CSI: Chemistry of Color.